A research group at Tohoku University has made a significant discovery with positive implications for the development of bacteria-fighting drugs. The aminoacyl-tRNA site (A-site) of the 16S RNA decoding region in the bacterial ribosome looks promising for a new era of antibiotic drug development.
Traditional aminoglycoside antibiotics are problematic given their high toxicity and the potential for resistance development. The research at Tohoku University focused on bacterial A-site binding small ligands whose structures are distinct from the aminoglycoside family, which offer potential for the development of novel drugs that treat bacterial infections with a reduction in the problems associated with traditional antibiotics.
The research group led by Dr. Seiichi Nishizawa and Dr. Yusuke Sato (Department of Chemistry, Graduate School of Science) has reported a novel small ligand, ATMND-C2-NH2 that has the tightest binding affinity for the bacterial A-site among the non-aminoglycoside ligands.
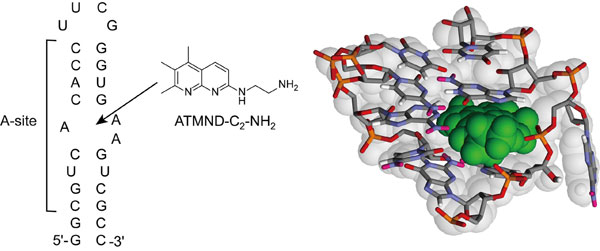
Chemical structure of ATMND-C2-NH2 and sequence of the bacterial (Escherichia coli) A-site-containing RNA model used in this study. It also shows the possible structure of the complex between ATMND-C2-NH2 (green color) and internal loop of A-site. Copyright:Seiichi Nishizawa.
ATMND-C2-NH2 shows a significant fluorescent quenching response upon selective binding to the internal loop of the bacterial (Escherichia coli) A-site-containing model RNA.
ATMND-C2-NH2 has also proven useful as an indicator for assessing ligand/A-site interactions.
The results obtained by the research group offer a rational basis for the generation of novel A-site binding ligands with a view toward novel antibiotics with less toxicity and minimum resistance development.
- Publication Details:
Title: Fluorescent trimethylated naphthyridine derivative with an aminoalkyl side chain as the tightest non-aminoglycoside ligand for the bacterial A-site RNA
Authors: Yusuke Sato, Masafumi Rokugawa, Sho Ito, Sayaka Yajima, Hiroki Sugawara, Norio Teramae and Seiichi Nishizawa
Journal: Chemistry-An European Journal
DOI: 10.1002/chem.201802320
Contact:
Yusuke SatoDepartment of Chemistry, Graduate School of Science, Tohoku University
Email: satoyuu
 m.tohoku.ac.jp
m.tohoku.ac.jpWebsite: http://www.anal.chem.tohoku.ac.jp/index_en.html