Researchers at Tohoku University have created a new catalyst that can partly renew itself while working, opening possibilities for more durable materials in energy and chemical applications. The catalyst is designed for the oxygen reduction reaction (ORR), an essential process for fuel cells and other clean energy technologies.
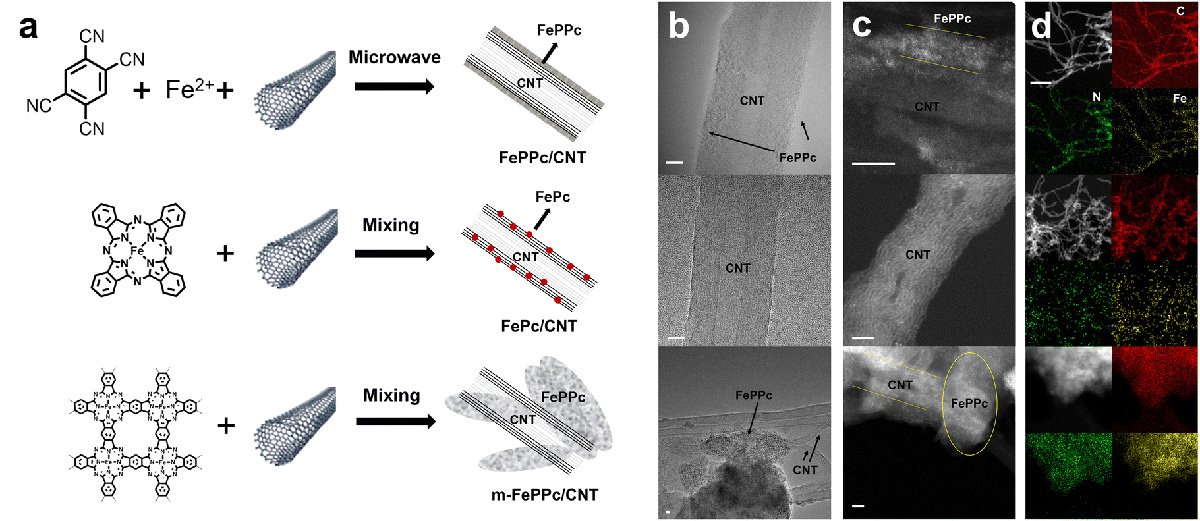
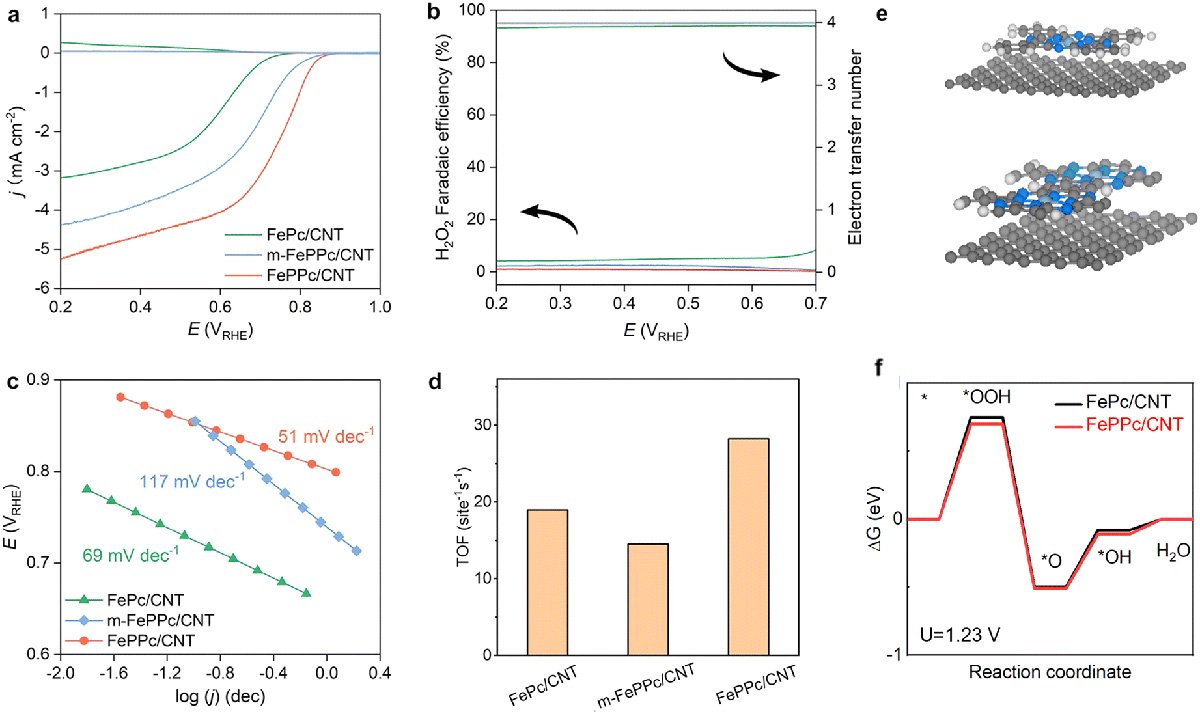
The new catalyst was made by forming a thin layer of iron polyphthalocyanine (FePPc) around carbon nanotubes. This method allowed more iron to be incorporated into the material than in earlier approaches, while still keeping the same type of active sites that drive the reaction. When tested in an acidic solution, the catalyst showed efficient performance in converting oxygen, with measurements indicating strong electron transfer and favorable reaction steps. The researchers traced this effect to the close interaction between the FePPc shell and the nanotubes.
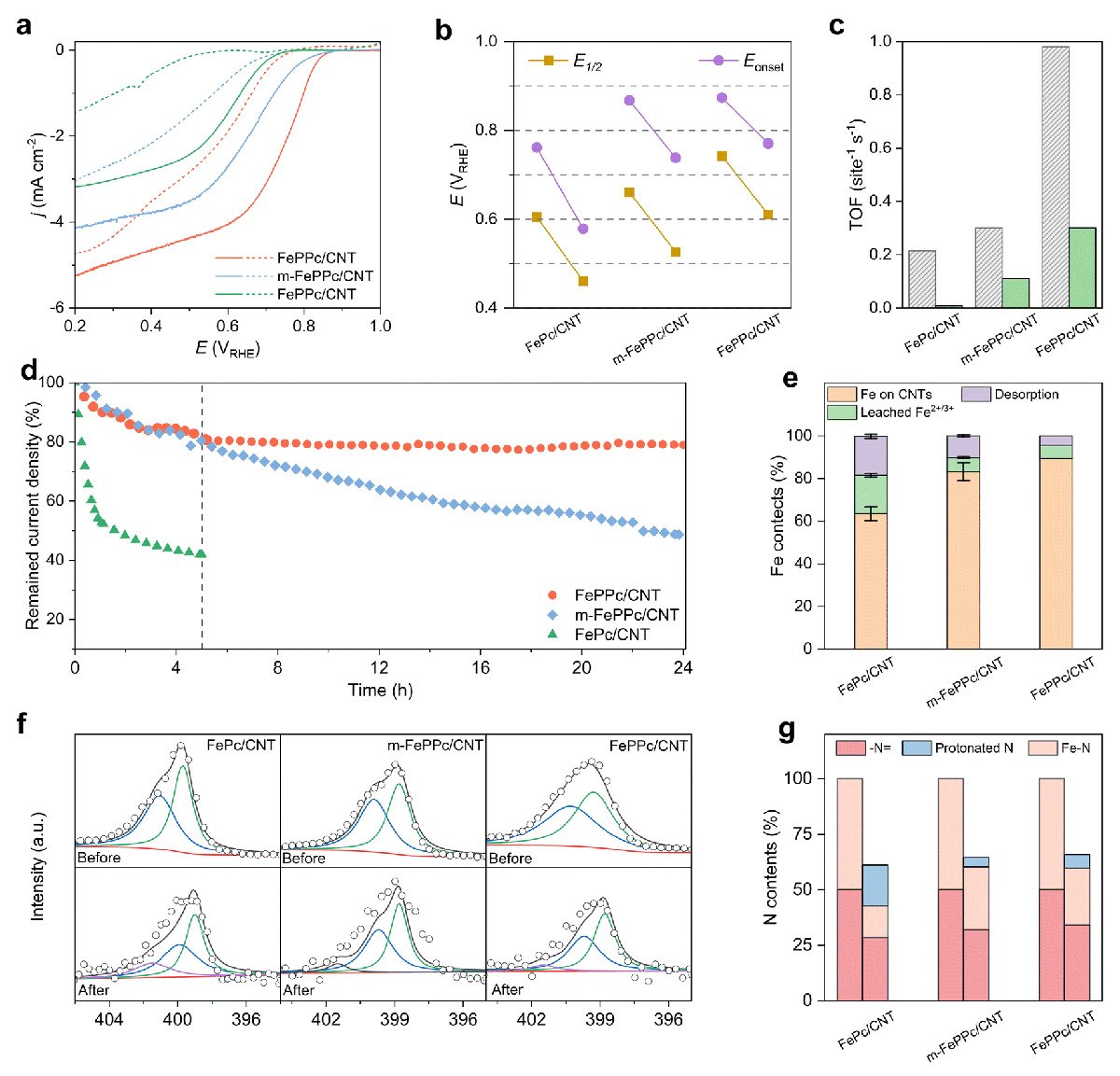
Just as important as performance is durability. In long-term testing, the catalyst kept around 80 percent of its initial activity after one day, whereas a similar material without the polymerized shell lost most of its activity in just a few hours.
The team discovered that the material changes as it works. Thin layers of FePPc gradually peel off, which exposes fresh active sites. At the same time, some of the peeled fragments continue to settle on the nanotubes, helping the reaction for a time. However, the process eventually reaches a limit as dissolved iron particles collect into stable clusters, which reduces the catalyst's effectiveness.
"Our results show that the apparent stability of the catalyst is linked to a balance between renewal of active sites and gradual processes of deactivation," explained Hao Li, a Distinguished Professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR). He added, "Understanding this dynamic evolution provides a framework to design Fe-N-C catalysts that remain effective under demanding electrochemical conditions."
By uncovering how this renewal process works, the study points the way toward designing more durable catalysts for clean energy devices and for the sustainable production of chemicals from renewable resources.
Details of the findings were published in the journal Energy & Environmental Science Catalysis on July 22, 2025.
- Publication Details:
Title: Dynamic evolution of self-renewal Fe-N-C catalysts for the acidic oxygen reduction reaction
Authors: Fangzhou Liu, Leo Lai, Zhongyuan Guo, Fangxin She, Justin Prabowoa, Hao Li, Li Wei and Yuan Chen
Journal: Energy & Environmental Science Catalysis
DOI: 10.1039/D5EY00092K
Contact:
Hao Li,
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
Email: li.hao.b8 tohoku.ac.jp
tohoku.ac.jp
Website: https://www.li-lab-cat-design.com/