A group of researchers has investigated whether data mining could accelerate the identification of low-cost metal oxide electrocatalysts, speeding up the world's transition away from fossil fuels.
Details of the research were published in the journal Advanced Science on December 7, 2023.
The world's dependence on fossil fuels has driven scientists to explore renewable energy sources. Electrochemical conversion technologies, such as fuel cell powering, water electrolysis, and metal-air batteries, offer promising strategies to transition towards a sustainable energy future. However, the reliance on precious metals in many electrocatalytic reactions poses economic and environmental challenges.
Metal oxides have the potential to change this owing to their stability and lower cost than precious metals, particularly under alkaline electrocatalytic conditions. Yet, the search for these metal oxides is resource intensive, with scientists relying on the trial-and-error process.
"With data mining a viable solution to this problem, we set out to probe the opportunities and challenges of adopting this strategy for finding metal oxides," says Hao Li, associate professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper.
To do so, Li, along with his colleagues, leveraged the extensive data available in the Materials Project database, identifying 68 promising stable metl oxide electrocatalysts under specific conditions.
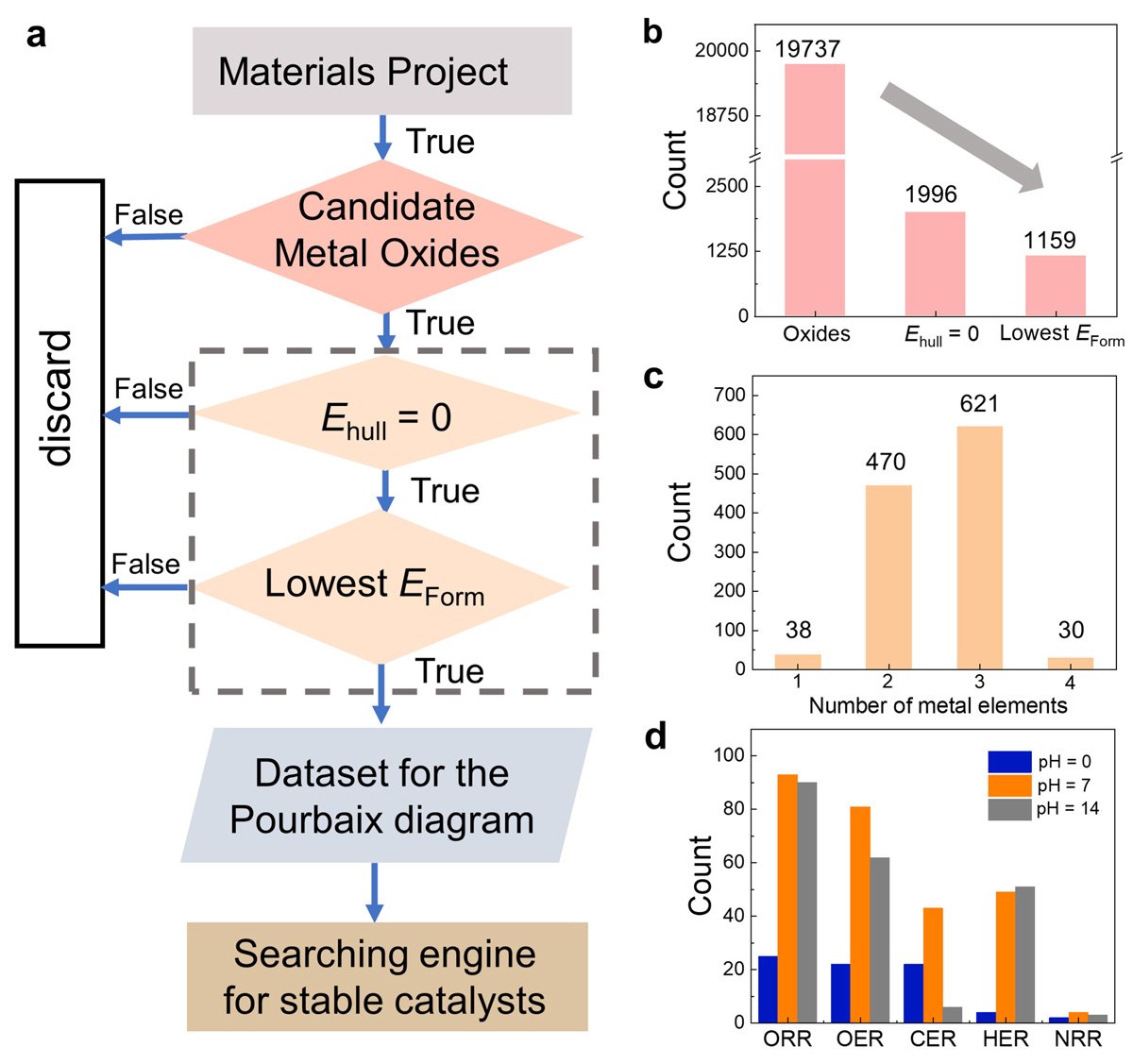
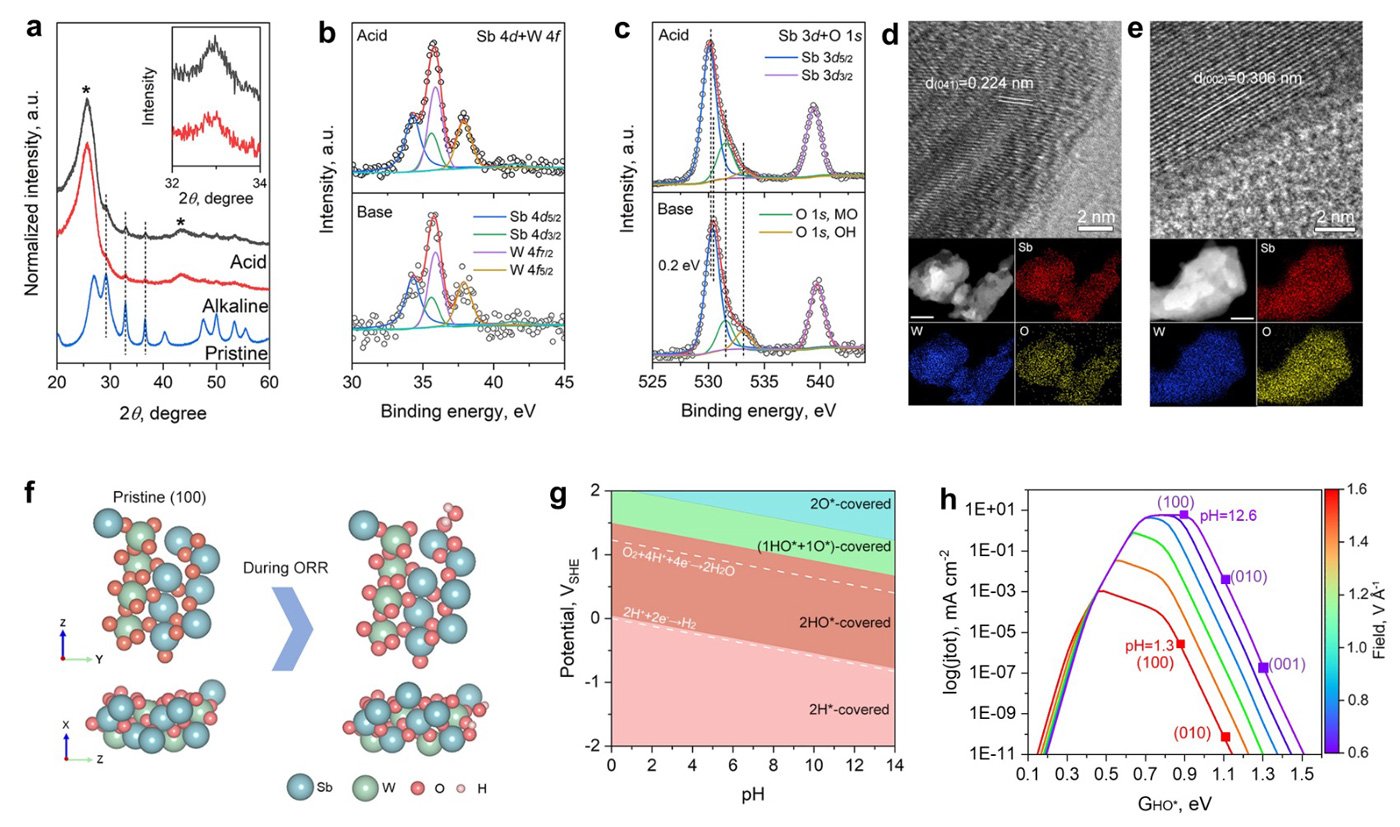
They noted that the database promoted Sb2WO6 as a stable metal oxide in acid. This is based on the aqueous stability diagram - a graphical representation that illustrates the thermodynamic stability of different chemical species in an aqueous solution as a function of pH and the electric potential. According to the diagram, Sb2WO6 is stable under the oxygen reduction reaction (ORR) in acidic media but rather unstable under high-pH ORR conditions. However, the researchers found that this contradicted subsequent experimental observations in alkaline ORR conditions.
Further post-catalysis characterizations, electrochemical surface state analyses, and pH-field coupled microkinetic modeling revealed that the Sb2WO6 surface undergoes electrochemical passivation under ORR potentials and forms a stable and 4e-ORR active surface.
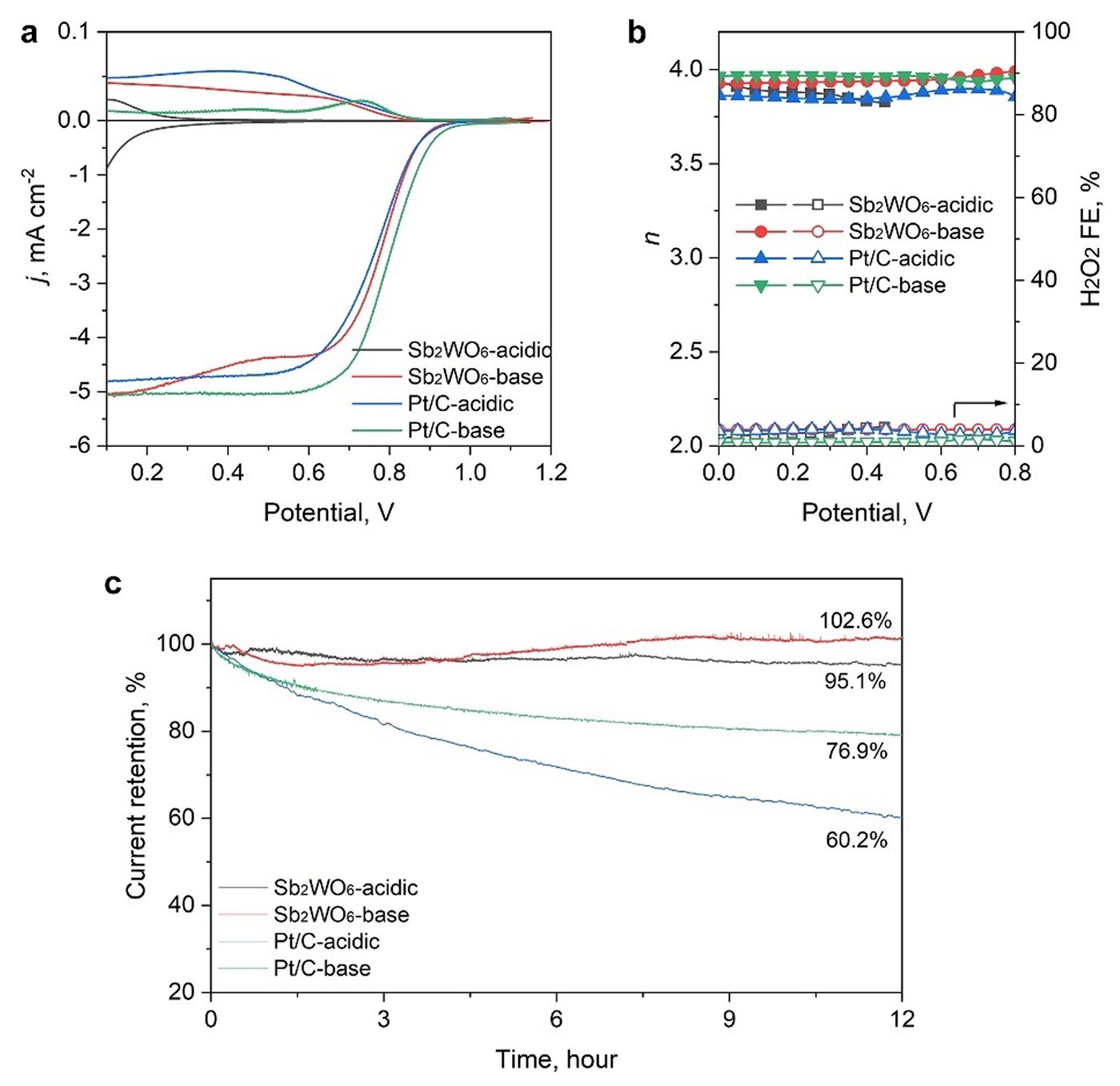
Overall, the study's findings indicate that while data mining holds promise, further refinement is necessary for widespread adoption. "A refined strategy needs to be developed that takes into account the electrochemistry-induced surface stability and activity," emphasized Li.
In the future, the researchers hope to explore other electrocatalysts for the oxygen evolution reaction and hydrogen evolution reaction by combining data mining, surface state analysis, and activity analysis.
- Publication Details:
Title: Identifying Stable Electrocatalysts Initialized by Data Mining: Sb2WO6 for Oxygen ReductionAuthors: Xue Jia, Zixun Yu, Fangzhou Liu, Heng Liu, Di Zhang, Egon Campos dos Santos, Hao Zheng, Yusuke Hashimoto, Yuan Chen, Li Wei, and Hao Li
Journal: Advanced Science
DOI: 10.1002/advs.202305630
Contact:
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
Email: li.hao.b8 tohoku.ac.jp
tohoku.ac.jp
Website: https://www.li-lab-cat-design.com/

