With potential high density, low cost, and a nature-friendly makeup, zinc-air batteries are touted to be the future of many energy devices.
Still, their low voltage of 1.4 V remains a barrier to more widespread usage, especially since lithium-ion batteries can generate 3.7 V. This limitation has meant zinc-air batteries are found mainly in electronic devices that need low power over extensive periods of time, such as hearing aids.
It is essential to improve the drive voltage and output power density in zinc-air batteries if they are to drive power sources for cutting-edge devices such as drones and electric vehicles. And now a research group has done just that, realizing a zinc-air battery with an open circuit voltage of over 2 V.
"We harnessed a cell with a rare-metal-free cathode and acidic/alkaline electrolytes arranged in tandem to overcome the major bottleneck for metal-air batteries," said Professor Hiroshi Yabu, corresponding author of the study.
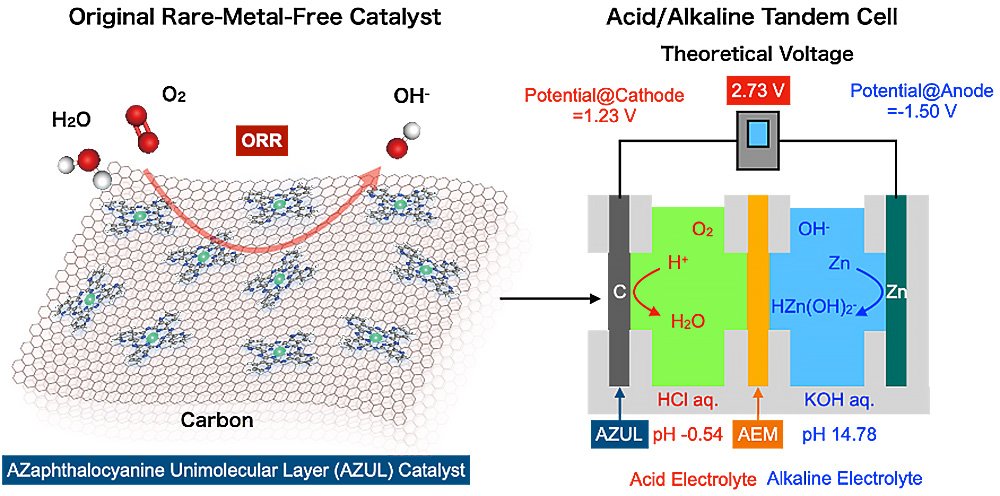
A battery's voltage is determined by the potential difference between the cathode and the anodes. The potential of the anode in zinc-air batteries involves zinc dissolving into the electrolyte, whereas the cathode potential concerns the conversion of oxygen's chemical energy into electrical energy, i.e., the oxygen reduction reaction (ORR).
Zinc-air batteries commonly used in hearing aids theoretically have a voltage of 1.9 V thanks to strong alkaline electrolytes. However, the ORR does not happen easily, and overvoltage is a common occurrence. Thus, output voltage stands at about 1.4 V.
Many scientists have sought to circumvent this by utilizing precious metal catalysts, such as platinum and carbon (Pt/C), due to their high ORR reaction activity. Yet these resources are both limited and expensive to use. Meanwhile, the reaction potential at each electrode strongly depends on the pH value of the electrolytes.
According to the Pourbaix diagram, which plots pH on the horizontal axis and reaction potential on the vertical axis, the dissolution potential of zinc is lowest under alkaline conditions, while the redox potential of oxygen is highest under acidic conditions.
"This led us to realize that by making the electrolyte on the zinc anode side alkaline, and creating acidic conditions on the cathode side, we could generate a higher voltage," added Yabu.
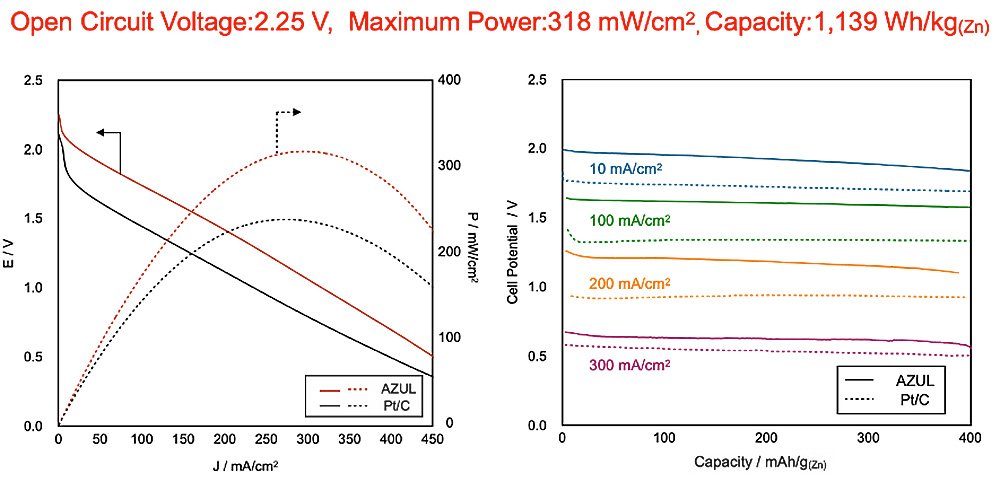
Details of the research were published in the journal APL Energy on April 24, 2023.
- Publication Details:
Title: Rare-metal-free Zn-Air Batteries with Ultrahigh Voltage and High Power Density Achieved by Iron Azaphthalocyanine Unimolecular Layer (AZUL) Electrocatalysts and Acid/Alkaline Tandem Aqueous Electrolyte Cells
Authors: Kosuke Ishibashi, Koju Ito, Hiroshi Yabu*
Journal: APL Energy
DOI: 10.1063/5.0131602
Contact:
Hiroshi Yabu
Affiliation: Advanced Institute for Materials Research
Email: hiroshi.yabu.d5 tohoku.ac.jp
tohoku.ac.jp
Website: http://yabulab.wp.xdomain.jp


