Stable and high oxide-ion conductors based on a new hexagonal perovskite-related oxide has been reported by scientists based at Tohoku University, Tokyo Tech, Kojundo Chemical Laboratory Co. Ltd. and the Australian Nuclear Science and Technology Organization (ANSTO) in a recent study. These high-performance oxide-ion conductors could pave the way for the development of solid electrolytes for next-generation batteries and clean energy devices such as solid oxide fuel cells.
The ever-increasing demand for clean energy and high-performance devices in the modern technological era has called for the development of alternate energy materials. In particular, oxide-ion conductors have garnered a lot of attention on this front. The presence of highly mobile oxide ions in their crystal structure imports unique electronic properties to these materials with potential applications in the design of solid oxide fuel cells (SOFCs), a promising technology for generating clean energy.
To develop efficient SOFCs, solid oxide-ion conductors with high conductivity and chemical and electrical stability are necessary. Unfortunately, conventional oxide-ion conductors do not show sufficient conductivity below 700 °C. An alternative material with high ion conductivity at lower temperatures (300 - 600 °C) is, therefore, highly sought after.
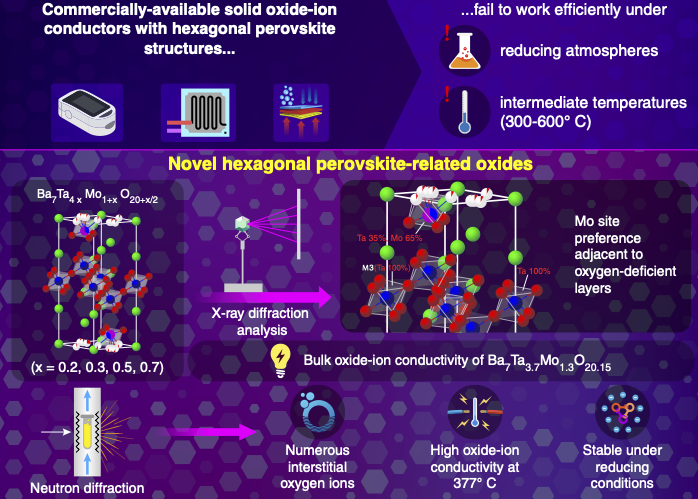
Fortunately, perovskite-type oxides could come to the rescue. In particular, hexagonal perovskite derivatives composed of barium (Ba), molybdenum (Mo), and niobium (Nb) oxides have been reported to exhibit high ionic conductivity. However, certain drawbacks still remain: the amount of oxygen in the interstitial spaces of the crystal structure, necessary for high conduction, is still low; electronic conduction competes with and hampers ionic conduction in a reducing atmosphere; and diffraction techniques are unable to shed light on the underlying oxygen migration mechanism.
In a recent study published in the journal Small, a team of researchers led by professor Masatomo Yashima from Tokyo Institute of Technology (Tokyo Tech) addressed these issues. The team developed a new hexagonal perovskite-related oxide, Ba7Ta3.7Mo1.3O20.15, which showed excellent ionic conduction at intermediate and low temperatures. "We aimed to design materials that allowed for the introduction of a large number of interstitial oxygens into their structure and showed high conductivity at intermediate and low temperatures. Additionally, the ion conduction remained dominant in a reducing atmosphere," elaborates professor Yashima.
High conductivity and stability in Ba7 Ta3.7Mo1.3Mo1.3O20.15will allow for the future development of solid electrolytes based on hexagonal perovskite-related oxides. ©Yashima et al.
The team then carried out structural analyses of the materials using a combination of synchrotron X-ray and neutron diffraction data and numerical calculations. They found that introducing tantalum (Ta) into the structure resulted in improved stability and a larger number of interstitial oxygens compared to the other hexagonal perovskite-related oxides. Additionally, the analyses and calculations showed that the Mo ions preferentially occupied the oxygen-deficient layers responsible for the oxide-ion conduction.
Co-author and assistant professor at Tohoku University's School of Engineering Taito Murakami carried out the electrical conductivity measurements, thermal analysis, and crystal structure refinement of this new oxide-ion conductor. "Our study demonstrates that hexagonal perovskite-related oxides have high potential as oxide ion conductors," said Murakami "We expect that this breakthrough will lead to future application in solid electrolytes such as fuel cells, gas sensors, and oxygen separation membranes."
- Publication Details:
Title: High Oxide-ion Conductivity in a Hexagonal Perovskite-related Oxide Ba7Ta3.7Mo1.3O20.15 with Cation Site Preference and Interstitial Oxide Ions
Authors: Taito Murakami, Toshiya Shibata, Yuta Yasui, Kotaro Fujii, James R. Hester, and Masatomo Yashima
Journal: Small
DOI: 10.1002/smll.202106785
Contact:
Taito Murakami,Department of Electronic Engineering
Email: taito.murakami.b7
 tohoku.ac.jp
tohoku.ac.jp


 Press release in Japanese
Press release in Japanese